These 'Metal-Organic Structures' Won A Nobel Prize – Now They Could Fight Climate Change
Since the Nobel Foundation's establishment at the turn of the 20th century, 1,026 recipients have been recognized by the prestigious award across six categories for their contributions to the advancement of mankind. The award has served as a barometer for scientific development, recognizing the world's brightest minds, from Marie Curie to Albert Einstein and Niels Bohr, for delivering some of the greatest discoveries of the modern age, including the X-Ray, Penicillin, in Vitro Fertilization, and the underlying technology behind the COVID-19 vaccine. 2025's recipients are no different, with honorees including visionaries in quantum mechanics, Hungary's master of the literary apocalyptic, and the orchestrators of immune system science who might break open man's understanding of autoimmune disorders and cancer.
But perhaps none of this year's recipients will have as significant an impact on mankind as the recipients of the Nobel Prize in Chemistry, whose development of metal-organic frameworks may be a game-changer for some of the world's environmental scientific challenges, ranging from climate change and sea pollution to drug delivery. Awarded to Susumu Kitagawa, Omar Yaghi, and Richard Robson, an international group of researchers at Kyoto University, UC Berkeley, and the University of Melbourne, respectively, the prize recognizes one of the most exciting developments in molecular chemistry: the invention of a new form of molecular architecture in which large pores between molecules, known as metal-organic frameworks, or MOFs, can capture and store substances, ranging from harmful substances like carbon dioxide or water pollutants to capturing water molecules in the desert air.
A game-changing discovery
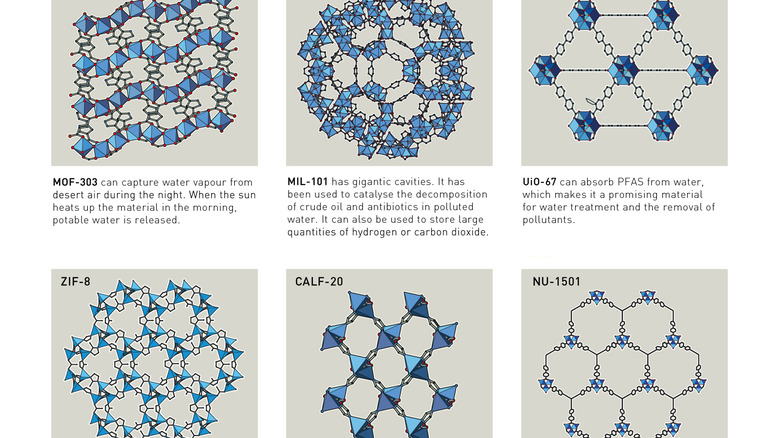
Put simply, metal-organic frameworks are crystalline materials formed of extremely porous networks of metal ions bound together by organic materials forming cavernous diamond-like structures. The high porosity of these structures is essential. Up to 90% of an MOFs is free volume, meaning one gram of powder — enough to fill up the space between your thumb and forefinger –– can possess more surface area than a football field. These vast internal spaces make MOFs more absorbent than zeolites and mesoporous silica and give MOFs a litany of applications, from storing gases to drug delivery.
The process started with Richard Robson in 1989, when he began experimenting with forming diamond-like molecular structures with copper ions. Made of pyramidal structures, these spacious, spongelike crystals, possessing innumerable large cavities, formed the building blocks for what would be a series of revolutionary discoveries. Since Robson's early experiments, both Kitagawa and Yaghi have expanded the possibilities of these molecular structures. Kitagawa, for instance, showed how gases could not only flow through MOFs but also do so without binding to the organic frameworks, making them easily removed from the structures. Yaghi, for his part, made the molecules more stable through new molecular links of carboxylate groups, allowing MOFs to withstand temperatures up to 300°C. Another advancement pioneered by Yaghi was expanding the surface area of MOFs up to 7,000 square meters per gram — a key development for applying MOFs to climate change, water shortages, and other key scientific quandaries. And while the technology may not be able to reverse climate change, metal-organic frameworks hold such immense potential that some scientists have dubbed them the material of the 20th century.
Applications
The rapid development of MOFs can be attributed to two major factors. The first is that MOFs are highly customizable structures. By changing the type of metal, composition of organic linkages, temperature, or solvent system, scientists can easily build new structures, crafting them for a variety of purposes. The second is that the field of metal-organic frameworks holds immense scientific and commercial potential. In fact, since the Nobel laureates' breakthroughs, chemists around the globe have created more than 100,000 types of MOFs.
One of the most important uses of MOFs is in gap absorption, in which MOFs capture greenhouse gases like methane, carbon dioxide, and nitrous oxide. without the need for burdensome or cost-prohibitive equipment. Another application is separating what scientists call "forever chemicals" from the world's water supplies. Known as PFAS, Per- and polyfluoroalkyl substances are synthetic pollutants that have polluted the earth's waterways and are extremely difficult to eradicate. Over the past few years, MOFs have become a promising solution to removing these harmful chemicals from water and soil. Another recent development is the use of MOF nets to harvest water from the desert air, pointing towards exciting hydropower and water conservation efforts. Beyond climate change, chemists believe MOFs can change a wide range of fields, including biomedicine, oil decomposition, horticulture, harvesting rare-earth elements, fluorescent sensors, and catalysis.
Despite these uses, developing MOF technology at scale has been slow. However, some see the technology as approaching an economic breakthrough, as lowered manufacturing costs, increased investment, and emerging technologies like artificial intelligence may help companies overcome the various bottlenecks that have typically stymied its commercial applications over the past three decades. According to a report by research firm IDTechEx, the MOF market may grow by 30x over the next decade, nearing the billion-dollar mark by 2035.