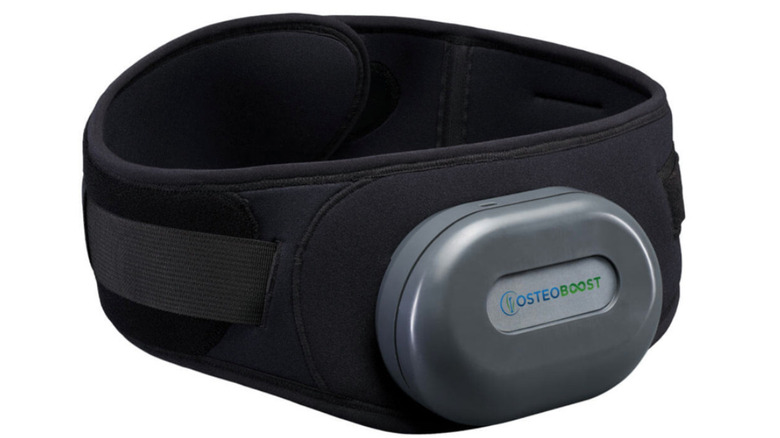
This 'Osteoboost' Belt Could Mean Big Things For Bone Health
As women age, preventing bone density loss becomes increasingly important, given that it has a direct correlation with fractures and a decreased quality of life. Previously, the treatment options for osteopenia — the precursor to osteoporosis — were limited to vitamin D, calcium supplements, and dietary modifications.
However, in a breakthrough development, U.S.-based Bone Health Technologies announced that the FDA granted clearance for Osteoboost — a wearable device that helps treat low bone density in postmenopausal women. Osteoboost is the first non-pharmacological device-based treatment available for postmenopausal women with low bone density.
The belt, which is to be worn around the waist, works by delivering targeted vibration to the hips and spine — two areas that are most at risk for low-bone-density-related breaks and fractures. The calibrated vibration helps reduce the decline of bone strength and density, and can even improve bone health among postmenopausal women diagnosed with osteopenia. This groundbreaking technology is medication-free, uses safe technology, and is straightforward to use, making it suitable for postmenopausal women across various age groups.
The need for Osteoboost
Bone Health Technologies, the firm that developed Osteoboost, reports that 52 million Americans suffer from osteopenia, a condition that is common in women over the age of 50. While it's not as severe as osteoporosis, osteopenia can eventually get to a more critical stage if it is not addressed in a timely manner.
"Today's groundbreaking decision represents the first non-pharmacological therapy approved to treat this widespread and serious condition. With Osteoboost, we have a new treatment option that taps into the body's natural mechanism to stimulate bone growth," states Laura Yecies, CEO of Bone Health Technologies. Since therapeutic options for osteopenia were previously limited, Osteoboost presents a significant advancement in the management and treatment of this condition.
Bone Health Technologies has highlighted that current treatment methods for osteopenia have low compliance rates. In contrast, the company reports that the compliance rate for Osteoboost is over 80%. This could be due to the belt's user-friendly design and the quick treatment regimen, which only requires the wearer to use the belt for 30 minutes a day. The piece of wearable tech also includes an auto-shutoff feature, which means wearers can go about their day as usual without having to take the belt off.
Clinical validation and sales of the device
Osteoboost received FDA clearance based on data from a double-blinded study that was conducted at the University of Nebraska Medical Center. The data was shared at the ENDO and ASBR conferences in 2023, and it helped demonstrate how successful Osteoboost is in preserving bone density and stimulating bone health and growth. The device's efficacy was measured by tracking vertebral strength, which was measured through CT scans. The subjects who used the device at least three times a week lost only an average of 0.48% bone strength, in contrast to the control group, which lost 2.84% bone strength.
Laura Bilek, Ph.D., Associate Dean for Research and Associate Professor at the University of Nebraska and the principal investigator for the study, confirms, "Although lifestyle interventions such as exercise and diet are beneficial to bone, the effect is small. The Osteoboost shows promise in slowing the loss of bone density and strength and may fill the treatment gap."
Osteoboost has received FDA approval, but it is not yet available for immediate purchase. If you're interested in purchasing the Osteoboost belt, you can add your details to the company's notification list to receive updates on its availability. And talk to your doctor in the meantime, as it is a prescription-only treatment option.