From Crude To Unleaded: How Gasoline Is Made
The story of gasoline as a potential fuel source begins back in 1859 when Edwin Drake tapped the first crude oil well in Pennsylvania. His most immediate use for the oil was the production of kerosene through a distillation process that converted the crude oil into something that could be used in lamps. That same distillation process created a bunch of other crude oil products, including gasoline.
Gasoline was originally discarded as a simple byproduct of kerosene production, and it wasn't until the arrival of the automobile that gasoline became the world's most well-known liquid fuel source. If you own a car — and it's not electric – then gasoline is likely a pretty regular purchase. You go to the corner store, pull your car up to the pump, stick the nozzle into the gas tank, and wait a few minutes. Then you're off!
You may not have thought much about how gasoline goes from the crude, sticky oil underground to a refined fuel moving you down the highways, but it's a pretty fascinating process once you start to get into it. This is how it happens.
Gasoline production takes millions of years
Our first introduction to crude oil may have happened only a century and a half ago, but nature has been busily cooking the raw materials for millions of years. Crude oil is, in the simplest terms, the leftover sludge of dead organisms when trapped under heat and pressure. It's called a fossil fuel for a reason — because it is quite literally dead creatures from eons past!
It's popularly said that oil is made of dead dinosaurs, but that's untrue. In fact, the lion's share of crude oil is made up of microscopic organisms like marine algae and plankton. When these teeny tiny plants and animals die and are not eaten, their bodies drift downward and collect on the seafloor. As they break down, the molecules of oxygen, nitrogen, phosphorus, and other organics get removed, leaving a layer of carbon and hydrogen behind.
If that layer of chemical sludge gets buried beneath sediment and exposed to heat and pressure, then those basic ingredients get transformed into a viscous hydrocarbon mixture known as petroleum or crude oil. The nature of the crude oil depends largely on how deeply the deposit is buried and how much heat and pressure is present, as it has to exist within a sweet spot where the pressure is high enough to create petroleum but not so high that the hydrocarbons are destroyed.
Crude oil
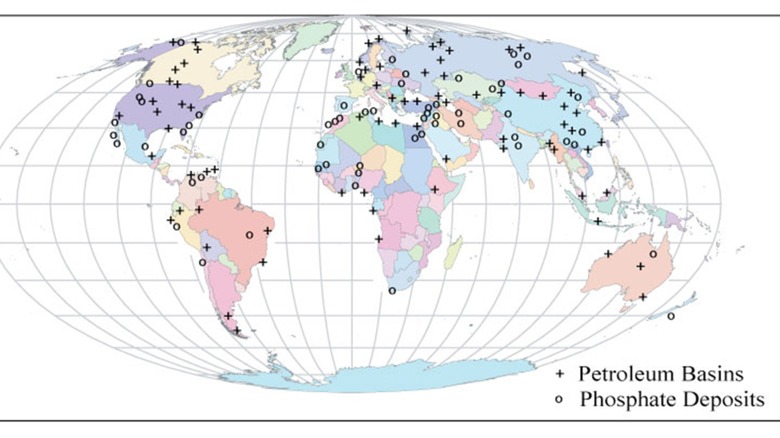
The common name for the liquified remains of those long-dead critters is crude oil, though you may also hear the name petroleum. Both terms are used more or less interchangeably. It is found in naturally occurring underground reservoirs all over the world. That's because, over the course of millions and billions of years, the Earth's organization has changed. Areas that are now landlocked were once underwater, meaning oil deposits can be found all over the place.
It's called crude because it is a disorganized mixture of many different hydrocarbon compounds, including paraffins, naphthenes, and more. While it is popularly portrayed as a thick black goo coming out of the ground, crude oil actually comes in a variety of forms depending on the mixture of hydrocarbons. Paraffins are typically the most common compounds found in any crude oil deposit and they are the major component of petroleum products like gasoline.
Finding an oil deposit
Have you ever wondered if you might be sitting on top of a pile of black gold? Maybe if you drilled into the ground beneath your house, you might find a pocket of crude oil just waiting to be pumped out and sold for profit. Unfortunately, that is probably not the case. Oil deposits don't form just anywhere — they need particular conditions, which means we can narrow down where they are likely to be.
In the old days, that meant using surface features to infer what might be going on below ground or using core samples to figure out the geological situation underfoot. Nowadays, we have some more advanced tools at our disposal. Drilling operations employ geologists to sniff out potential oil deposits and tell them where to drill, and they do that in a number of ways.
First, geologists consider what we know about the planet's history. Using models of the planet's past, they can figure out where organic materials were likely to be deposited and where they might be found today. Core samples are still used to find the right kind of rock, but geologists largely rely on seismic data to find underground oil deposits, which is possible because seismic waves move through different materials at different rates. By shaking the ground and listening to how it jiggles, scientists can create a map of underground materials — kind of like a planetary ultrasound — and see the oil beneath.
We've got a live one!
Once geologists have identified a promising location, it's time to drill, but it's not as simple as punching a hole in the ground and sucking up the liquid. Once a pocket is tapped, the natural pressure of the deposit is enough to push crude oil to the surface. In the old days, miners would chip away at the rock with a chisel-shaped drill attached to a lever. As the lever moved up and down, the drill chipped away at rock which would then have to be removed manually before drillers could continue.
When drillers finally pierced the pocket, the pressure would send oil rocketing through the tiny hole, a phenomenon called "blow-outs." That's the source of the famous image of a freshly drilled oil well shooting crude oil into the sky like a broken sprinkler. These events are not only dangerous, they're also inefficient. If you're going to all the trouble of drilling a well, you want to capture as much of the oil as possible.
These days, specialized drills are used to cut through rock while also depositing a compound known as drilling mud. The mud serves two main purposes. The first is to act as a cork, holding pressure inside the pocket so oil doesn't burst out. The second is to pull freshly drilled rock to the surface so it can be removed without having to stop the drill. This way, wells can be drilled more quickly and without losing oil to the surrounding environment.
Oil transport
Once it's pulled out of the ground, crude oil has to get to refineries all over the world, where it can be turned into gasoline and other petroleum products. The thing is, prospecting improvements have led to wells springing up in pretty inhospitable places.
Deposits have been found on every continent except for Antarctica. That's because commercial drilling in Antarctica is forbidden as the process of extracting the oil in that environment would be both dangerous and expensive, which means that there's not a lot of motivation to go looking for oil there. Meanwhile, petroleum products are needed in every corner of the globe. Consequently, the transportation of crude oil is important and complicated.
Petroleum first has to get from the well site to a refinery. Then, the products have to get from the refinery to their final destination in your gas tank. Pulling that off requires a huge network of oil tankers, barges, railroads, trucks, and pipelines. Basically, every common mode of transportation — with the major exception of air travel — is used to transport crude oil around the globe.
What is oil refining?
When crude oil comes out of the ground, it is a mess of jumbled hydrocarbons, and while it will readily burn as is, the raw extracted oil is not all that useful until it has been refined. You can think of crude oil as something like a baked cake you're trying to unbake. Sure, you could just eat the cake if that's what you want, but you have more options if you can separate it into flour, eggs, milk, etc. Cake is great, but it's not always what you need.
There are about 20 gallons of gasoline inside each 42-gallon barrel of crude oil, but to get our hands on it, we have to separate it from all the rest. In order to turn crude oil into a gallon of gas or a water bottle, it has to be sorted into its various components. In simple terms, this is a process of refining, which means isolating the elements you want and getting rid of the rest. The same term is often used in metallurgy to discuss the purity of a metal, and the same philosophy is at work here. Only, in this case, we're talking about the purity of hydrocarbons.
How oil refining works
Inside the crude oil there are certain hydrocarbons that can become gasoline, while other hydrocarbons are good for other types of fuel or other petroleum products. Each of those components needs to be isolated and collected. Oil refineries achieve that through a distillation process that is similar to the one used in the creation of alcohol. The secret is heat.
Hydrocarbons all have different boiling ranges depending on their weight. Lighter hydrocarbons like butane begin to separate at a lower boiling range while heavier compounds like kerosene evaporate at temperatures above 300 Fahrenheit. The refinery heats crude oil until most of it evaporates and floats up a distillation column. What's left behind is a thick sludge of the heaviest hydrocarbons.
Since various hydrocarbons evaporate and condense at different rates, a refinery can boil them off and collect them at different points along the distillation column. Evaporated hydrocarbons travel up the column and recondense as a liquid at specific heights. Those liquids, now known as fractions, are collected and are more or less ready for market.
Breaking and reforming
The distillation process refines most of the crude oil in each barrel, but you don't always end up with the petroleum products you want. Sometimes, you have too many light fractions and not enough heavy ones, so some remixing is needed. Even when that's not the case, you're usually left with chemically heavy hydrocarbons called gas oils at the bottom of the distillation column. While gas oils aren't useless, they aren't as valuable as gasoline, so refineries are motivated to make them lighter. Taken together, refineries need a way to make light hydrocarbons heavier and heavy hydrocarbons lighter.
Gas oils can be converted into lighter hydrocarbons through a process known as cracking. They are placed inside a reinforced reactor which applies heat and pressure while introducing catalysts to break heavy hydrocarbon molecules into lighter ones. Once broken, the lighter molecules have a lower boiling point and can be distilled in the usual way. Meanwhile, if a refinery has an excess of light hydrocarbons like naphtha, they can be combined with other molecules inside reformers to make heavier hydrocarbons suitable for use in gasoline and other fuels.
Mixing
Hydrocarbons come out of the distillation column in different streams and with different qualities, and multiple streams might get mixed together in order to end up with the gasoline that is ultimately shipped to a gas station near you. Mixing or blending determines, among other things, the grade (amount of octane) in the gasoline and which nozzle it ends up coming out of.
Mixing or blending is also a way of maximizing profit for a refinery by ensuring the greatest number of products meet the required specifications. For instance, after a batch of crude oil has been distilled, a refinery might end up with one stream that exceeds specifications and another stream that fails to meet them. By mixing the two streams together, you get two streams that both meet the requirements.
As we're working with raw materials that include natural variation, the end result is correspondingly variable. Blending is a way to ensure that the end product is as consistent as possible by mixing different streams with compatible inconsistencies. It's the Goldilocks philosophy of fuel production, wherein the combination of streams results in products that are just right.
Gasoline byproducts
While no two barrels of crude oil are the same, roughly 42% of each barrel will ultimately become gasoline, on average. Another 27% becomes diesel fuel, meaning that nearly three quarters of each barrel makes its way to the gas pump in one form or another. About 6% of each barrel becomes jet fuel, 5% becomes tar-like heavy fuel, 3% becomes light fuel, and 2% becomes other hydrocarbon fuels.
After all of the fuels have been removed, you're left with about 14% of the original barrel. About 4% of that will become asphalt used to make roads and sidewalks. The last 10% gets spread around to just about every industry on the planet and is where we get petroleum products from plastics to perfumes and everything in between.
It's likely that you're surrounded by petroleum products, not just now, but always. In fact, the device you're using to read this is made, in part, from oil pulled out of the ground. Petroleum is used to produce antifreeze, car tires, clothing, fertilizers, paint, soap, yarn, and more. It's also used to make almost every plastic product you've ever used. Every water bottle, plastic shopping bag, and single-use eating utensil was once a hot, pressurized glob of hydrocarbons buried in the earth. On a long enough timeline, it might be again.