FDA Committee To Rule On Experimental Alzheimer's Drug That Could Slow Disease
The FDA is holding an early hearing today on a new Alzheimer's treatment, aducanumab, the first new drug for the disease that the US Food and Drug Administration has considered giving the green light to since 2004. The panel, a meeting of the FDA's Peripheral and Central Nervous System Drugs Advisory Committee, will aim to decide whether or not recommendations for approval should be given for the experimental Biogen drug.
Even if the Committee decides the answer to that is yes, that's not to say whether the FDA will finally grant aducanumab approval for public use. A final decision from the Food and Drug Administration on that could also take some time, potentially waiting until 2021.
Trials of aducanumab have met with mixed results. The drug focuses on reducing amyloid beta, which Biogen says is implicated in the onset and development of Alzheimer's disease. While administration of the drug may not cure it, aducanumab could potentially "delay clinical decline in patients," the pharma company suggests.
Almost six million people in the US currently suffer from Alzheimer's disease, which typically manifests in symptoms of memory loss, a reduction in ability to carry out daily tasks and activities, and behavioral changes. As dementia continues to build, patients are left entirely dependent on their care-givers. Ultimately, it is fatal.
Exactly what causes – and can accelerate or slow – Alzheimer's is still the subject of considerable research. Biogen's strategy is to challenge the amyloid beta which is a precursor to so-called neurofibrillary tangles. These later bind to synapses in the brain, disrupting and then destroying them completely.
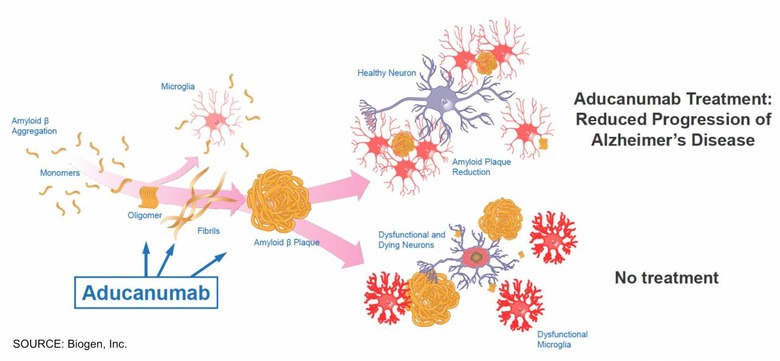
While there are existing therapies to treat Alzheimer's, aducanumab would – if approved – be the earliest to be applied. Intended to be administered during the earlier stages of disease, it could slow the progression of the neurofibrillary tangles. The evidence, however, hasn't been entirely conclusive.
A Phase 3 clinical trial was ended in early 2019, for example, with Biogen conceding that the drug was unlikely to reach its goals. A subsequent trial, however, proved more successful. Aducanumab, Biogen said, contributed to a significant reduction in clinical decline among Alzheimer's patients, "with robust and internally consistent results."
It was followed by an application to the FDA for approval, but before that can be decided, today's Committee meeting must give their opinion. The FDA itself is under no obligation to accept that judgement, and indeed there are other concerns beyond the early indicators of some clinical efficacy. Skeptics point to the limited pool of testing as one issue, along with the overall cost of the proposed treatment regimen.
Biogen hasn't said exactly how much it would expect aducanumab to cost for public use, though estimates pegging the drug in the thousands of dollars or more aren't unbelievable. Combined with the intended early prescribing, that could be an obligation too far for either the FDA or insurers to stomach.