The FDA Rules Just Changed On COVID-10 Monoclonal Antibody Treatments
The U.S. Food and Drug Administration (FDA) has just released a new update in regards to the treatment options for COVID-19. As a result of the update, the FDA has removed the authorization for two monoclonal antibody treatments. Starting January 24, 2022, these treatments will only be authorized in certain regions.
In the statement posted on FDA's website, more information is revealed concerning these treatments and the reasons why they are no longer authorized. The affected monoclonal antibody treatments include two mixes of medications: bamlanivimab and etesevimab, which are given together, and REGEN-COV, which is comprised of casirivimab and imdevimab. As FDA explains in its post, monoclonal antibodies are proteins made to copy the immune system's ability to combat various harmful pathogens, including viruses such as SARS-CoV-2.
Although these treatments have shown some promise with previous variants of the coronavirus, it seems they are ineffective against the currently rampaging Omicron variant. For that reason, the FDA is choosing to limit the use of these treatments to only include patients who may have been infected with another strain of the virus. In the United States, that's a very short list of people.
Omicron variant requires other treatments
The FDA stated that it chose to revise the authorization for these monoclonal antibody treatments because "[they] are highly unlikely to be active against the Omicron variant." As they come with a host of side effects (via UPMC), including allergic reactions, the FDA concluded that there is no reason to expose the patients to these treatments — unless the person in question is infected by a different variant of COVID-19.
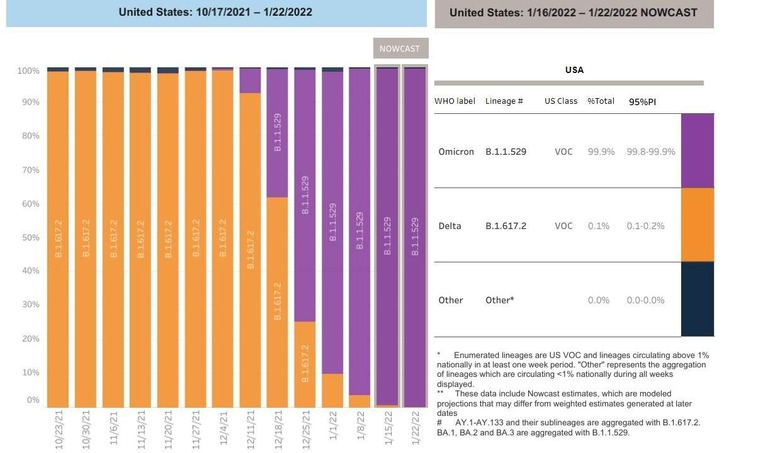
According to the CDC, the Omicron variant now constitutes 99.9% of all confirmed coronavirus cases in the United States. The Delta variant is all but gone (0.1%,) and other variants are non-existent. This means that monoclonal antibodies would be ineffective for 99.9% of infected individuals, and if they are ineffective against the Omicron variant, it makes perfect sense for the FDA to move on to other forms of medication.
The authorization for these forms of treatment may not be gone forever. The FDA promises that in the event of an outbreak of another variant of the novel coronavirus, it may once again authorize the use of monoclonal antibodies — provided that they are proven to be effective against the new strain.
Will Omicron be the end of the pandemic?
Fortunately, Omicron patients are not left without treatment due to this change. The FDA lists Paxlovid, sotrovimab, Veklury (remdesivir,) and molnupiravir as possible treatment options that have proven to be effective against Omicron. These options are used to treat patients with mild-to-moderate COVID-19 infections. At the same time, the FDA continues to stress the best way to stay protected is to get vaccinated and refresh the effects of the vaccine with a booster shot.
As we are closing in on two years of the pandemic in the United States, many are asking themselves whether Omicron will finally be the end of the pandemic. Statements from medical professionals and scientists are all over the media, with sometimes very contradictory opinions surfacing in these interviews. Although the spread of Omicron has been rapid and it has left a lot of people with active antibodies in its wake, it's impossible to tell whether that will be enough to slow down the pandemic and bring the world back to normal.
Many choose to be cautious in their verdicts. Dr. Anthony Fauci said in an interview on Radio Davos, "It is an open question as to whether or not Omicron is going to be the live virus vaccination that everyone is hoping for." Meanwhile, the World Health Organization urges the public not to assume that the Omicron variant will bring an end to the pandemic, and to stay cautious in the midst of it (via Reuters).