New Material "Superior" To Graphene Could Unlock Breakthrough Batteries
A new carbon network, similar to graphene but with a far more complex microscopic structure, could lead to better electric vehicle batteries researchers have predicted. Graphene, arguably the best-known exotic form of carbon, has already been tapped as a potential game-changer in li-ion battery tech, but new manufacturing methods could eventually produce even more power-dense cells.
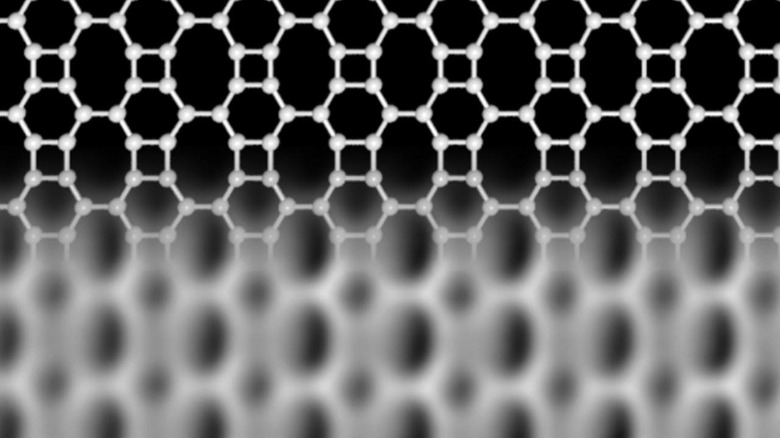
Graphene is basically a mesh of carbon atoms, in which tiny hexagons are created as each links to three neighbors. However researchers have theorized that other structures could be generated too, beyond this straightforward honeycomb.
That's what a team from the University of Marburg in Germany and Aalto University in Finland have developed, once again tapping carbon atoms but coaxing them into new orientations. The so-called Biphenylene network that is the result is made up of hexagons, squares, and octagons, a more complex mesh than graphene forms. As a result, it has distinctly different – and in some ways preferable – electronic properties, the researchers say.
For example, while graphene is prized for its ability to act as a semiconductor, the new carbon network behaves more like a metal. At just 21 atoms wide, indeed, stripes of Biphenylene network can function as conducting wires for electronic devices. At that scale, graphene is still behaving like a semiconductor, they point out.
"This novel carbon network may also serve as a superior anode material in lithium-ion batteries, with a larger lithium storage capacity compared to that of the current graphene-based materials," Qitang Fan, of the University of Marburg, and lead author of the new study, suggests.
Lithium-ion battery anodes typically comprise of graphite layered onto a copper foil. It's highly conductive, which is essential for not only reversibly placing lithium ions between its layers, but also because it can continue to do that for potentially thousands of cycles. That makes for both an efficient battery but also one that lasts extended periods without degrading.
An even more efficient, smaller alternative based on this new carbon network, however, could make for denser cells. That might allow for EVs and other devices using li-ion batteries to be smaller and lighter.
As with graphene, though, figuring out how to manufacture this new version at scale is the next challenge. The current assembly method relies upon a super-smooth gold surface, onto which carbon-containing molecules are initially formed into chains of linked hexagons. A subsequent reaction then links those chains, forming the squares and octagons that distinguish the end-result from graphene.
"The new idea is to use molecular precursors that are tweaked to yield biphenylene instead of graphene," Linghao Yan, of Aalto University, explains. The goal is to now produce larger sheets of the material, so that its properties can be better understood.