Self-Assembly Process Builds Nanowires With Tiny 3-Atom Wide Copper-Sulfur Crystalline Core
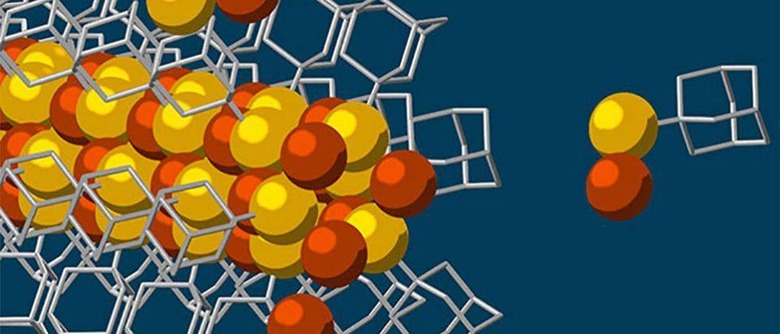
Stanford University researchers with help for the US Department of Energy's SLAC National Accelerator Laboratory have created a new self-assembly process that that uses something called diamondoids to create tiny nanowires that have a solid core. That solid core inside the tiny nanowires is made from a 3-atom wide copper-sulfur crystalline material and is the smallest core possible. The tiny nanowires have superior electrical properties due to the lack of defects in the solid crystalline core.
The self-assembly process opens the door for new developments in optoelectronic devices and superconducting materials. "Achieving a 'solid core' of a three atom cross section is ideal," says Nicholas Melosh, an associate professor at SLAC and Stanford, in an e-mail interview with IEEE Spectrum. "It's small enough to exhibit unique functionality, yet it can tolerate single defects or strains since there is still a pathway for the electrons to flow."
The self-assembly process that the team created starts with diamondoids, the smallest form of diamonds and in this usage these diamondoids are just interlocking carbon and hydrogen atoms. The diamondoids are attracted to each other through something known as van der Waals forces that exist between molecules or groups of atoms. The assembly process starts with all ingredients added to a single pot and then sulphur is added to the mix.
Those sulphur atoms attach to the diamondoids and then the sulfur atom bonds with a single copper ion and forms the basic building blocks for the nanowires. The van der Waal forces then draw the building blocks together to grow the nanowire. "Other molecular self-assembly methods have been tried, yet balancing the delicate interplay between attractive and repulsive forces to get just the size you want has proven very difficult," said Melosh.
SOURCE: Spectrum