Insulin Syringes Recalled Across The Globe Over Risk Of Injecting Wrong Dose
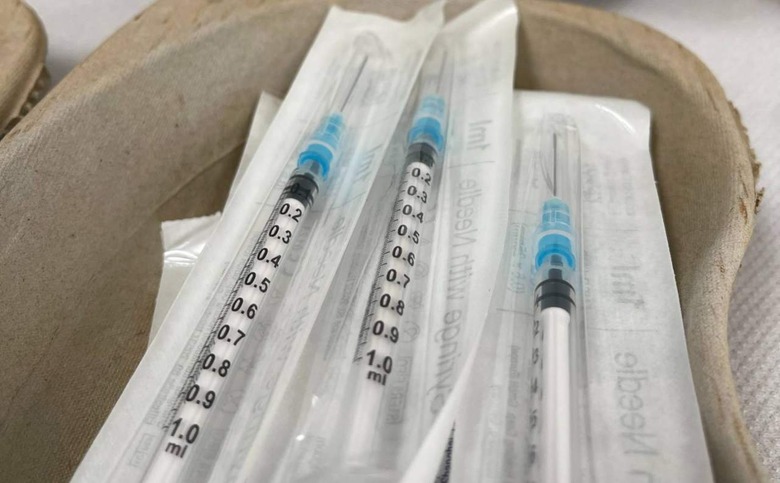
Multiple batches of fixed-needle insulin syringes have been recalled around the world over the potential risk of skewed lines on the syringe barrels. This issue, according to the recall notice, may result in some diabetic patients injecting too much or too little insulin for their needs, potentially putting them at risk of severe adverse effects as a result.
The new recall comes from Smiths Medical, which notes that the US Food and Drug Administration has labeled this a Class 1 recall. That designation means the agency believes there's a 'reasonable probability that the use of or exposure to' the recalled product may result in 'serious adverse health consequences or death.'
According to the company, it learned that some lots and models of its Jelco Hypodermic Needle-Pro Fixed Needle Insulin Syringe products may feature "skewed" odd-number line markings on the syringe barrels. These line graduation markings are off by around 20-degrees, Smiths Medical says, which may result in some diabetics injecting the wrong insulin dose.
If a patient injects too little insulin, they may develop hyperglycemia (high blood sugar) which can lead to issues like ketoacidosis. On the other hand, if a patient injects too much insulin, it may cause their blood sugar levels to drop too much, leading to anything from mild symptoms like chills and anxiety to serious outcomes like seizures and death.
Fortunately, the company says that it hasn't received any reports of serious injuries or death as a result of the recall syringes. Consumers who may have one or more of these syringes should check the item's packaging and compare its lot numbers with the numbers provided in the recall notice.