FDA Says Lidocaine Numbing Product Recalled Because It's Too Potent
Pharmaceutical company Teligent has recalled some of its topical lidocaine numbing product due to 'super potency,' meaning it is stronger than anticipated. This super potency can, according to the recall notice, result in an issue known as local anesthetic systemic toxicity, which itself comes with multiple potential issues.
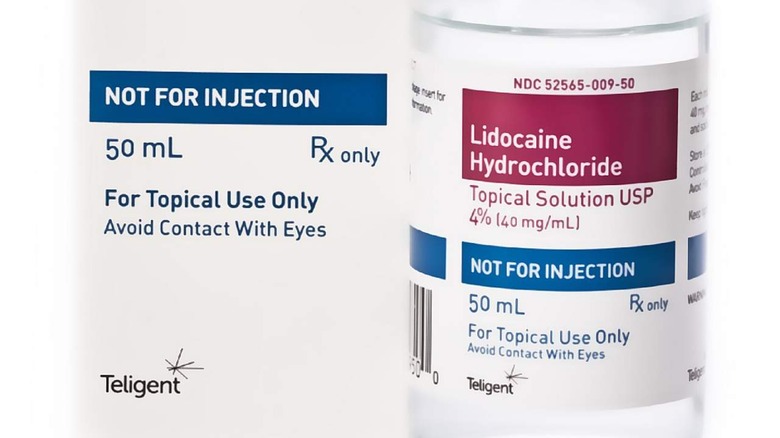
The recall covers one lot of 50ml glass bottles containing lidocaine HCl topical solution 4%. Teligent Pharma explains that testing at "the 18-month stability timepoint" found the recalled lidocaine to be super potent. Using the topical lidocaine with super potency could lead to local anesthetic systemic toxicity.
If that were to occur, the recall advisory explains that someone may experience various severe health complications that, if not treated in time, could lead to severe outcomes and possibly death. The issues include a drop in blood pressure, depression or excitation of the central nervous system, and cardiovascular collapse.
According to the pharmaceutical company, the recalled product is packaged in glass bottles featuring screw caps. Users can identify the recalled items by looking at the NDC number, lot number, and expiration dates listed in the recall published on the FDA's website.
Any consumers who have one of the recalled lidocaine bottles are told to return it to the place of purchase; the company is alerting its distributors to get the products sent back. Teligent says the recalled lidocaine product was distributed in both the United States and Canada.