Bottles of lidocaine sold for pain relief recalled over super potency
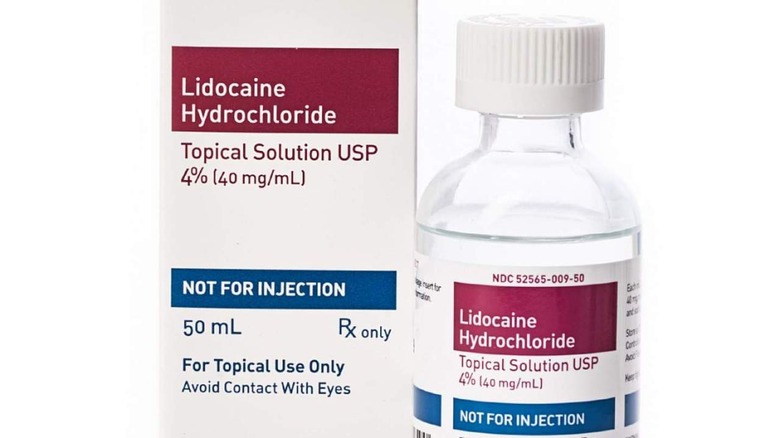
Teligent Pharma has announced a new recall that impacts its Lidocaine HCl Topical Solution USP 4% anesthetic liquid sold in 50ml glass bottles. The reason, the company explains, is the potential for "super potency," meaning the bottles may contain more lidocaine than listed on the label. This, in turn, puts users at risk of toxicity.
Lidocaine is an anesthetic often sold as the main ingredient in numbing products, including sprays used to help reduce sunburn pain, creams used to reduce burn pain, and gels intended to reduce tooth pain. Though these products, including similar ones that contain benzocaine, are available over-the-counter in the US, they typically contain only very low levels of the anesthetic due to toxicity risks (via FDA).
More potent anesthetic products may be made available to certain patients through prescriptions, however, for things like numbing a nasal passage before inserting a feeding tube (via NCBI). One such prescription-only numbing product is Teligent Pharma's Lidocaine HCl topical solution.
The liquid is sold in a glass bottle with a screw cap and — based on testing — may be too potent. The super potency was detected at the nine-month and 18-month stability timepoints, according to Teligent, which says its product is intended for use as topical anesthesia in the "oral and nasal cavities and proximal portions of the digestive tract."
Too much lidocaine and toxicity
Teligent Pharma explains that using a product that contains too much lidocaine may result in a higher than intended dose. As a result, it is possible the patient could develop a condition called local anesthetic systemic toxicity (via NIH), which may impact the central nervous system and cardiovascular systems. Failure to recognize and treat the toxicity could, in some cases, lead to death.
In light of these risks, Teligent Pharma has recalled the impacted lidocaine bottles and is arranging to have them returned from distributors. Any patients or consumers who may be in possession of a recalled bottle are advised to stop using it immediately and instead return it to the store from which it was acquired for a refund.
Should you be worried?
It's important to note that while pain relief products are sold over the counter, including some numbing solutions for issues like tooth pain, the bottles covered by this recall were only available through prescription. If you purchased a bottle of liquid anesthetic or other over-the-counter numbing product, it's likely not the same one recalled by Teligent.
To make sure, check the product you own for the brand, manufacturer, and other identifying details. Two lot numbers are covered by Teligent's recall, one with an expiration date of 05/2023 and another of 01/2024. The recall notice has been published on the FDA's website, including a picture of the product's label (above), to help consumers determine whether they have one of the recalled bottles.
If you do own one of the recalled bottles, it is very important to check the recall advisory, follow the recall instructions, and contact the company and/or one's healthcare provider for additional information.