3D Graphene Is Lighter Than Steel But Ten Times Stronger
Yes, that is almost an oxymoron but almost a material scientists' dream come true. A material that has 10 times the strength of steel, one of the hardest man-made materials, and yet also just 5% of its density. But that is precisely what researchers at MIT might have just accomplished by taking graphene, believed to be the strongest materials in existence, and forming it into a structure that resembles a coral more than a bar of steel.
Graphene, a form of carbon, may be considered the strongest, but like any superhero, it has one fatal flaw. It is only two-dimensional. Yes, graphene is a two-dimensional material, basically only one atom thick. Try using that for your next house. What these MIT researchers did, then, was to heat and compress flakes of graphene into a 3D structure.
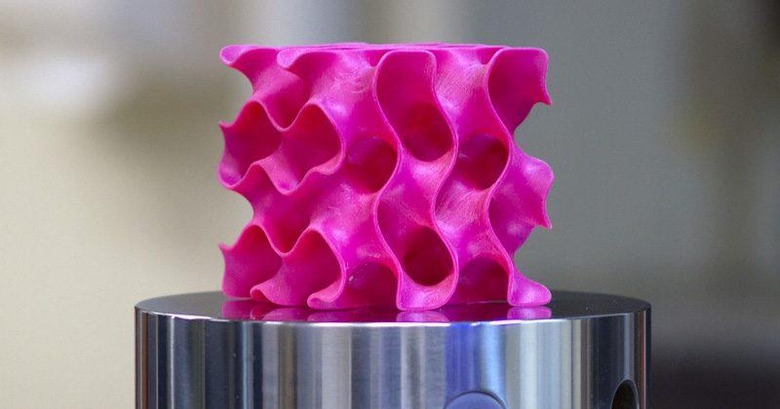
But that alone wasn't really enough. The secret sauce to this discovery was actually the shape of the "3D graphene" itself. When heated and compressed, graphene naturally took the form of a ball full of holes, whose formal name is a gyroid. This gave scientists the idea that it's not exactly the nature of the material used but the shape it takes.
To test that theory, they used those gyroid forms, magnified 1,000 times, to 3D print plastic sponge-like structures that varied in minor details, like, for example, thickness. Using their new data models, they were able to, more or less, accurately predict how much pressure these structures could take before breaking apart. Curiously, the one with thicker walls ended up an explosive mess sooner than the thinner one.
It's probably going to be a while before we actually see graphene structures of that size being used to build things, but the research paves a new way to form extra strong structures. Any material can be used in place of graphene and would still benefit from the strength due to the odd and porous shape it takes. And that porous form could also lend the structure easily to other uses, like filtration systems or processing of liquids.
SOURCE: MIT