Antarctica's Bleeding Glacier: The Blood Falls Phenomenon Explained
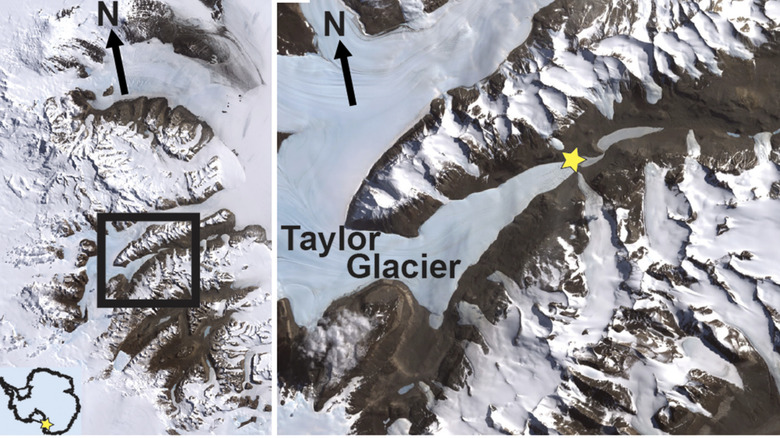
The ruddy red "waterfall" known as Blood Falls was first found by a party of geologists led by Thomas Griffith Taylor during the Terra Nova expedition (1910-1913). These geologists made up the western party of the expedition, while Robert Scott and his team made their ill-fated race to the South Pole. The falls are located on what's now known as Taylor's Glacier, an area of Antarctica almost directly south of New Zealand.
Though it's surrounded by typical icy whiteness, the liquid rushing forth is a striking rusty red. The cause of this phenomenon was a mystery when the falls were discovered, with the first guesses suggesting red algae in the water might have been affecting the color. When it was described chemically in the 1960s, scientists realized it was the high iron content of the water that was creating the color, but they couldn't figure out its source or how it traveled through the glacier in a liquid state.
In 2017, however, a study was published in the Journal of Glaciology that finally put the mystery to rest. Using radio echo sounding to map the water feeding the falls, a team of scientists discovered a hydrological system of lakes and ponds that were trapped underground millions of years ago.
How the hydrological system was formed
Today, the bodies of water within Taylor's Glacier both sit on top of ice and are completely covered by ice. Being sandwiched between layers of ice in a -17 degree Celsius environment, you might wonder how the water hasn't turned to ice itself. Well, a lot of it did. About five million years ago, flooding in the eastern areas of Antarctica formed these salty pools of water on the continent's surface. Around three million years later, temperatures dropped low enough that glaciers began to form on top of the lakes and pools.
As more and more liquid turned to ice on the surface of the lakes, the salt was left behind in the remaining water, increasing the salt concentration until it eventually became a brine. Because brine has a lower freezing point than pure water, the ancient lakes remained as a pocket of liquid within the glacier. Another two million years later in the present day, the top layer of Antarctic ice is now 400 meters thick, and the salt content in the brine lakes is three times that of seawater.
How the brine travels through the glacier
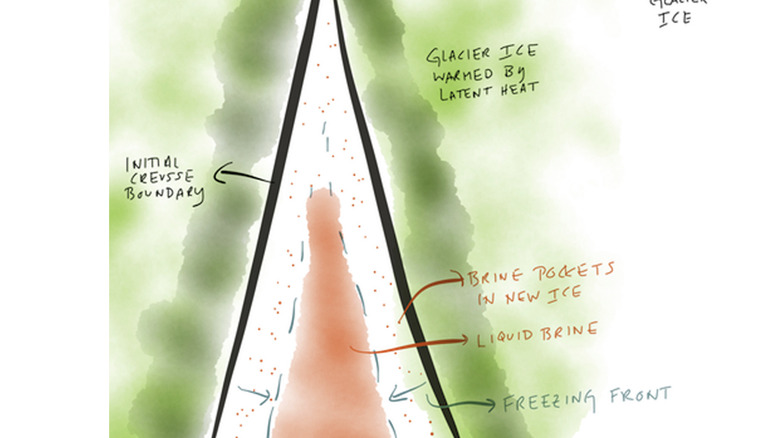
The brine's high salt content isn't the only thing that keeps it from freezing. When water or brine freezes, it releases a hidden form of energy known as the latent heat of freezing. Trapped underground with no light or oxygen, this latent heat can warm surrounding brine or melt surrounding ice. This phenomenon is what helps the brine travel through the glacier. As the glacier moves, cracks and crevasses form throughout and those that open near the brine lakes fill with pressurized brine.
The temperature drop causes the brine to begin to freeze, releasing latent heat and warming the surrounding ice. This makes new crevasses more likely and eventually results in the formation of pathways that allow the brine to travel. Complex glacial movements and resulting hydraulic potential gradients then push the brine toward a specific exit — Blood Falls. Once the brine spurts out of the glacier and meets oxygen for the first time, it instantly oxidizes and turns a distinctive shade of red.
Where all the iron comes from
So that explains how the lakes got there, why they don't freeze, and how they're traveling through the glacier and out of Blood Falls. There is still one question left, though — where is all the iron coming from? It seems there are two sources of iron entering the subglacial lakes, the first coming from Antarctica's bedrock. Iron is one of the most common elements on Earth, and there's plenty of it in bedrock that Taylor's Glacier is constantly scraping up against. These movements cause iron to enter the hydrologic system and affect the chemical makeup of the brine lakes.
Secondly, despite the extremely cold, extremely dark, extremely salty, and entirely oxygen-free nature of the brine lakes — they sustain life. Specifically, they are home to a type of microbe that can convert sulfur and iron compounds into energy, also known as chemosynthetic bacteria. These bacteria eat away at the bedrock to obtain energy, slowly eroding it and causing even more iron to enter the brine. Of course, since there's no oxygen in the underground lake system, the brine isn't red while it's in there. It's only once the brine finds its way to the surface that it oxidizes and turns red.
To put it more succinctly...
To wrap things up, here's a more condensed version of the tale:
-
Five million years ago, floods in Antarctica created a number of lakes, ponds, and pools on the surface. Three million years later, glaciers began to form on top of the lakes.
-
As the surface froze, it left the salt behind and increased the concentration of the remaining water, lowering its freezing point and allowing it to exist under the ice as a liquid.
-
A mixture of physical erosion from glacier movement and chemical erosion from unusual microbes caused large amounts of iron from Antartica's bedrock to enter the brine lakes.
-
Glacial movement also created cracks for the brine to enter, where latent heat kept it in liquid form and hydraulic potential gradients pushed it along pathways and out of Blood Falls. As the brine spills out, it oxidizes and appears a rusty red color.