Glucagon fibril structure discovered by MIT researchers
Researchers at MIT have been conducting research that could help people who have type 1 diabetes. People with that medical condition have to inject themselves with insulin to allow their body to absorb sugar from the bloodstream. The human body has another hormone called glucagon that has the opposite effect.
Glucagon can be given to a diabetic patient to revive them if they become unconscious due to a severe hypoglycemia. Glucagon given to patients now is powdered and has to be dissolved in liquid before injecting. The reason is that the hormone tends to form clumps called amyloid fibrils if it is stored as a liquid. A new study from MIT has revealed the structure of those glucagon fibrils and suggests possibilities for altering the amino acid sequence so the protein gets less clumped.
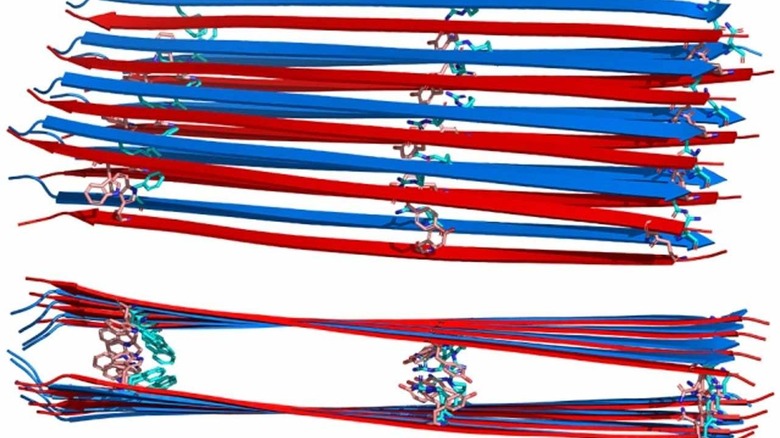
The MIT team says that insulin is stable for many weeks in a solution, and the goal is to achieve the same stability with glucagon. The study used nuclear magnetic resonance (NMR) spectroscopy to discover that the structure of glucagon fibrils is unlike any other amyloid fibrils of known structure. Scientist on the project say that glucagon is an "alpha helix" inside the body that binds tightly to a liver cell receptor causing a reaction that releases glucose into the bloodstream.
When glucagon is dissolved into a solution at high concentration, it transforms into fibrils within hours. Using NRM, the team found that glucagon fibrils consist of many layers of flat sheets, known as beta sheets, that are stacked on top of each other.
Unlike other amyloid fibrils with known structures, the peptides run antiparallel to each other. That means that each strand run in the opposite direction from the other two on each side of it. The peptides from a 10nm long beta strand, which is the longest beta strand known so far among any protein. To stop clumps from happening, you will need to change the identity of the amino acid according to the team. Researchers are working on ways to modify the sequence to break the stabilizing interactions to make the peptide no longer assemble into a fibril.